UW & Fred Hutch Research Teams Publish Findings on MCC Immunology



In two new studies published this month in Cell Reports Medicine, scientists from the UW School of Medicine and Fred Hutchinson Cancer Center have found that the presence of cancer-fighting immune cells in a patient’s blood is the best predictor of whether Merkel cell carcinoma (MCC) tumors will respond to immune checkpoint inhibitors.
Immune checkpoint inhibitors (ICI) help release molecular brakes that allow cancer-killing immune cells to fight MCC. The findings are a step toward the development of a clinical test that could someday guide MCC treatment.

These two studies highlight the importance of patients having pre-existing cancer-specific T cells circulating in the blood when ICI therapy is initiated. With these cancer-fighting T cells poised to go to work, releasing the breaks allows for them to immediately target the cancer. Ongoing studies by this group are investigating the potential use of a clinical tool that leverages this biomarker to stratify which patients are likely to respond well to ICI and which need alternate or combination therapy.

Scientists from the UW Department of Dermatology involved in this research include Paul Nghiem, MD, PhD, Principal Investigator for the Nghiem Lab, Saumya Jani, MD/PhD student, Thomas Pulliam, MD/PhD student, Candice Church, PhD, Senior Research Scientist, and Rima Kulikauskas, Senior Research Scientist.
Congratulations to both research teams on identifying this significant key to understanding which patients will respond to immune checkpoint inhibitors and insights into how we can help improve responses!
Study 1: Circulating cancer-specific CD8 T cell frequency is associated with response to PD-1 blockade in Merkel cell carcinoma
Highlights
-
Frequency of anti-tumor CD8 T cells at baseline in blood predicts anti-PD-1 response
-
Frequency of anti-tumor CD8 T cells in the tumor does not predict anti-PD-1 response
-
Intratumoral CD8 T cells are more dysfunctional than their peripheral counterparts
-
HLA-I downregulation is a mechanism of secondary resistance to anti-PD-1 in MCC
Summary
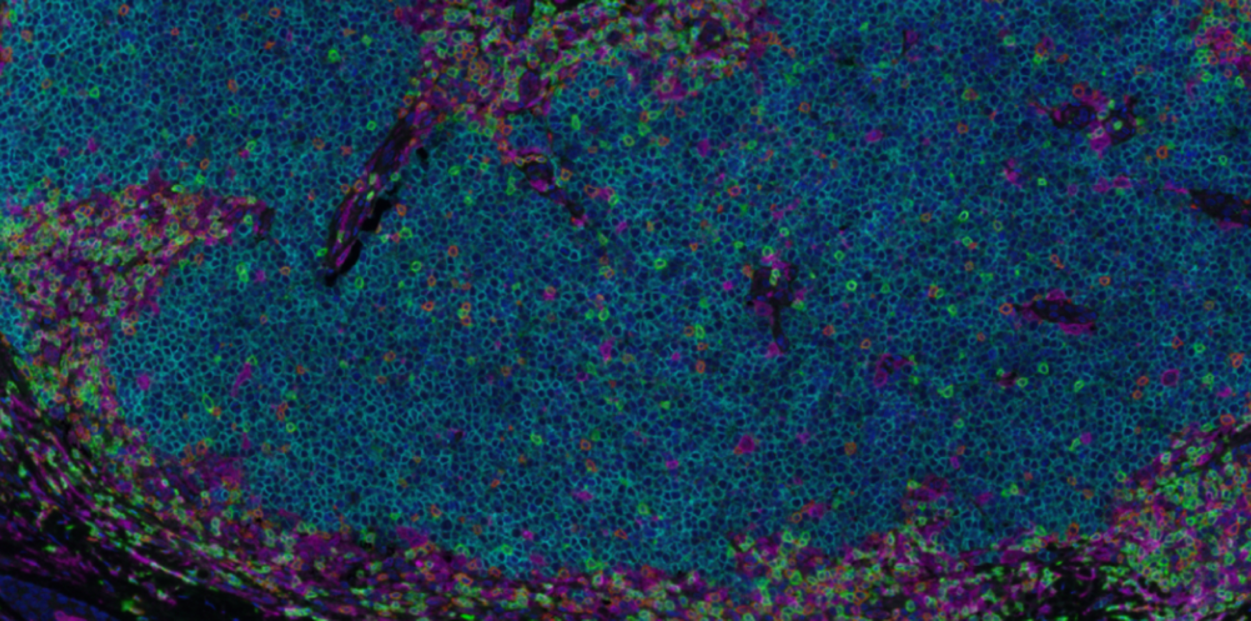
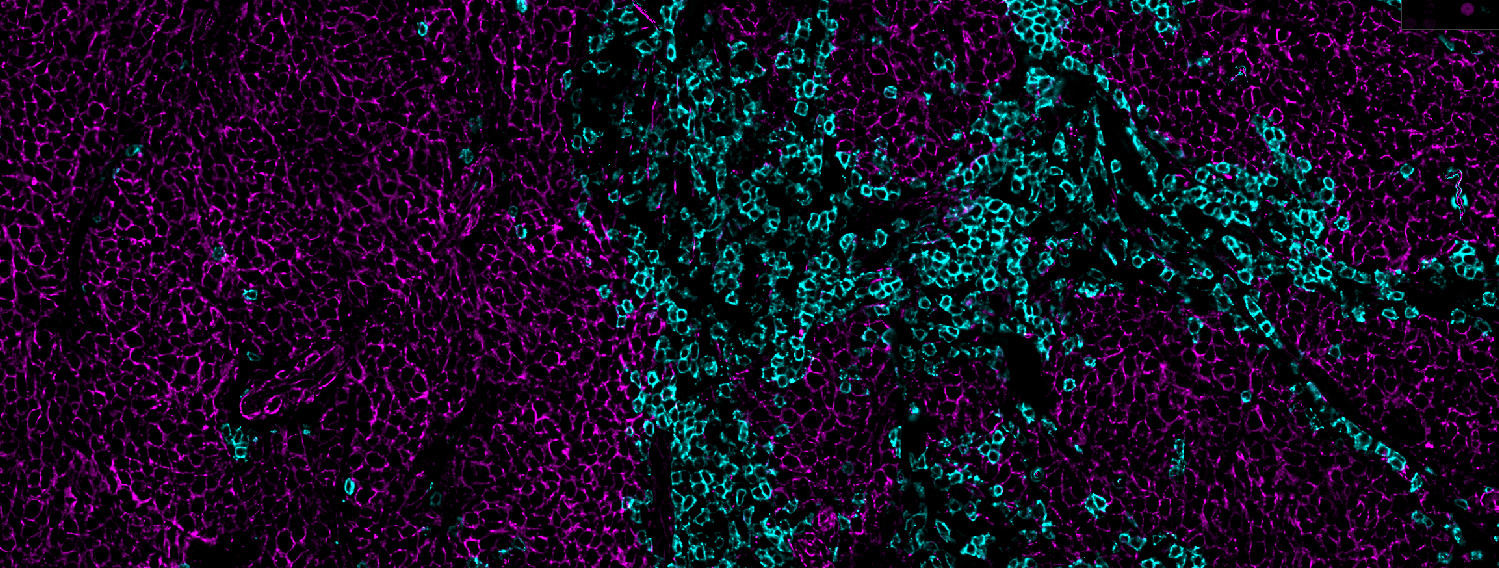
Understanding cancer immunobiology has been hampered by difficulty identifying cancer-specific T cells. Merkel cell polyomavirus (MCPyV) causes most Merkel cell carcinomas (MCCs). All patients with virus-driven MCC express MCPyV oncoproteins, facilitating identification of virus (cancer)-specific T cells. We studied MCPyV-specific T cells from 27 patients with MCC using MCPyV peptide-HLA-I multimers, 26-color flow cytometry, single-cell transcriptomics, and T cell receptor (TCR) sequencing. In a prospective clinical trial, higher circulating MCPyV-specific CD8 T cell frequency before anti-PD-1 treatment was strongly associated with 2-year recurrence-free survival (75% if detectable, 0% if undetectable, p = 0.0018; ClinicalTrial.gov: NCT02488759). Intratumorally, such T cells were typically present, but their frequency did not significantly associate with response. Circulating MCPyV-specific CD8 T cells had increased stem/memory and decreased exhaustion signatures relative to their intratumoral counterparts. These results suggest that cancer-specific CD8 T cells in the blood may play a role in anti-PD-1 responses. Thus, strategies that augment their number or mobilize them into tumors could improve outcomes.
Study 2: Merkel cell polyomavirus-specific and CD39+CLA+ CD8 T cells as blood-based predictive biomarkers for PD-1 blockade in Merkel cell carcinoma
Highlights
-
MCPyV-specific CD8 T cells in blood predict MCC patient response to anti-PD-1
-
Blood MCPyV-specific CD8 T cells often express CD39, CLA, or CD103
-
Bulk CD39+CLA+ CD8 T cells predict response to anti-PD-1
-
Bulk CD39+CD103+ CD8 T cells correlate with tumor burden and predict poor outcomes
Summary
Merkel cell carcinoma is a skin cancer often driven by Merkel cell polyomavirus (MCPyV) with high rates of response to anti-PD-1 therapy despite low mutational burden. MCPyV-specific CD8 T cells are implicated in anti-PD-1-associated immune responses and provide a means to directly study tumor-specific T cell responses to treatment. Using mass cytometry and combinatorial tetramer staining, we find that baseline frequencies of blood MCPyV-specific cells correlated with response and survival. Frequencies of these cells decrease markedly during response to therapy. Phenotypes of MCPyV-specific CD8 T cells have distinct expression patterns of CD39, cutaneous lymphocyte-associated antigen (CLA), and CD103. Correspondingly, overall bulk CD39+CLA+ CD8 T cell frequencies in blood correlate with MCPyV-specific cell frequencies and similarly predicted favorable clinical outcomes. Conversely, frequencies of CD39+CD103+ CD8 T cells are associated with tumor burden and worse outcomes. These cell subsets can be useful as biomarkers and to isolate blood-derived tumor-specific T cells.